Abstract
Introduction: Daratumumab is a first in class immunoglobin G1 kappa human monoclonal antibody that targets the CD38 antigen on the exterior of myeloma cells. Daratumumab provides an important treatment option and has become standard of care for patients with relapsed/refractory multiple myeloma (MM). While generally well tolerated, daratumumab is associated with a high incidence of IRRs (42-56% of patients; grade 3/4: 2-9%) (Palumbo A et al. New England Journal of Medicine 2016; 375: 754-766, Voorhees P et al. Blood 2015; 126: 1829, Lonial S et al. The Lancet 2016; 387: 1551-60). These reactions predominately occur during the first two infusions. The manufacturer recommends both pre- and post-medications for daratumumab, including steroids, antipyretics, and antihistamines with or without other supplementary agents. Dana-Farber Cancer Center (DFCI) implemented an augmented pre- and peri-infusion medication regimen in November 2015, meant to reduce the occurrence of daratumumab-associated IRRs (table 1).
Methods: We conducted an observational, retrospective, single center, medical chart review of MM patients who received at least one dose of daratumumab between November 2015 and October 2017 at DFCI. Data were collected on patient characteristics (age, gender, number of previous treatment regimens, immunoglobulin subtype, and baseline disease burden), pre-/peri-/post-medications, daratumumab infusions (infusion duration and dose), and IRRs (grade of reaction and treatment of reaction). Data was collected for the first two daratumumab infusions (Cycle 1 Day 1 and Cycle 1 Day 8 (C1D1 and C1D8) for each eligible patient. The primary objective was to determine the incidence of IRRs in patients treated with daratumumab post-implementation of an augmented pre- and peri-medication regimen. Secondary objectives were to determine the infusion time of daratumumab and to characterize the use of infusion pre-and peri-infusion medications in patients receiving daratumumab at DFCI.
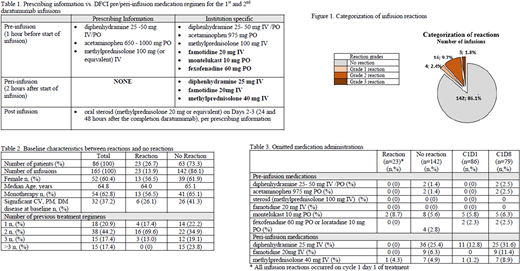
Results: A total of 105 patients who received ≥1 dose of daratumumab during the 2-year study period were identified. Eighty-six patients met inclusion criteria and received a total of 165 evaluable infusions. Twenty-three of the 86 patients (26.7%) experienced any grade IRRs. All IRRs were ≤ grade 3 in severity (grade 1-2: 23.2%; grade 3: 3.5%). Figure 1 highlights categorization of IRRs in the study population based on the total number of infusions. IRRs were managed by supportive care medications and/or briefly stopping the infusion per prescribing information and institutional practice. All reactions occurred during the first dose of daratumumab. The differences in baseline characteristics between the patients with and without reactions are outlined in table 2. More patients that received combination therapy experienced an IRR compared those on monotherapy (65.1 vs 56.5%). Patient comorbidities did not appear to influence IRR incidence (26.1% without IRR vs 41.3% with IRR). Of the patients that received >3 previous treatment regimens, none experienced an IRR. The mean duration of infusion for daratumumab was longer in patients who experienced an IRR compared with those who did not (505.9 vs 355.3 minutes). Seventy-one pre- and peri-infusion medications were omitted during the study period, representing a 95.2% adherence rate to the 9 medications contained within the institutional standard regimen. Details on omitted medications can be found in table 3. Peri-infusion diphenhydramine had the highest incidence of omission and was not given in 36 (21.8%) daratumumab infusions. Rates of medication omission were higher during C1D8, where no IRRs were observed.
Conclusions: An augmented regimen of pre- and peri-medications appears to lower the incidence of grade 1-2 IRRs with daratumumab, compared to previously published data. The results also support that most IRRs occur during the first dose of daratumumab. This suggests the benefit of an augmented pre- and peri-medication regimen for at least the first dose of daratumumab.
Richardson:Amgen: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal